
Email:[email protected]
Tel:+86 13482208428
EN


Feb 01,2021
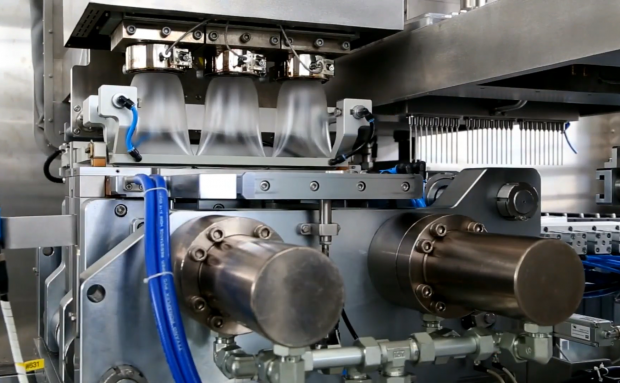
The biggest advantage of BFS technology is to prevent external pollution-human interference, environmental pollution, material pollution, etc. Aseptic fillingThermoforming, filling and sealing are fully automated and produced in a rapid continuous cycle (about 11-15 seconds), minimizing the contact with the external environment.The three-in-one technology is an automatic process mode. The BFS equipment blows thermoplastic materials…
Feb 01,2021
The biggest advantage of BFS technology is to prevent external pollution-human interference, environmental pollution, material pollution, etc. Aseptic fillingThermoforming, filling and sealing are fully automated and produced in a rapid continuous cycle (about 11-15 seconds), minimizing the contact with the external environment.The three-in-one technology is an automatic process mode. The BFS equipment blows thermoplastic materials…
Jan 18,2021
The Preparation System That Meets The BFS Process Requirements Should Also Have The Following Functions:(1) Good airtightness. To prevent contamination after sterilization, the system should be stored under positive pressure and airtight conditions(2) Good pressure resistance, in order to ensure the sterilization temperature in the system SIP, a certain pressure must be maintained(3) High temperature…
Jan 12,2021
BFS technology has long been used in liquid pharmaceutical products, used to form and fill containers ranging from small ophthalmic ampoules to large containers of IV solution. More recently, BFS technology has been expanding into injectables and into biologics, including vaccines and monoclonal antibodies. BFS technology is an indispensable technology for packaging liquids and semi-solids…
Jan 04,2021
Blow filling (Fill) sealing (Seal) three-in-one process (abbreviated as BFS process) is an aseptic packaging process invented by the engineering OO Gerhard Hansen of the German Rommelag Group in the 1960s . This process enables the three operations of bottle making, filling and sealing to be completed in the same station under aseptic conditions. The…
Dec 03,2020
The preparation system that meets the BFS process requirements should also have the following functions: (1) Good AirtightnessTo prevent contamination after sterilization, the system should be stored under positive pressure and airtight conditions. (2) Good Pressure ResistanceIn order to ensure the sterilization temperature of the system SIP, a certain pressure must be maintained. (3) High…